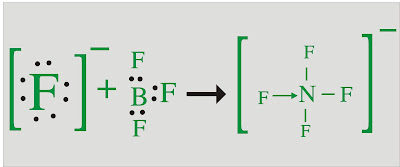
BF3:
Central atom is B. There are 3 fluorine atoms.
Valence electrons = 0
Negative Charge is = 0
Add all these, 3+3+0 = 6
Number of Bond (N/2) = 6/2 = 3
Table 1 tell us bond that it is a Triangular Planner.
BF4- :
Central atom is B. There are
4 fluorine atoms.
Valence electron = 3
Negative charge is = -1
Add all these, 3+4+1 = 8
Number of bond (N/2) = 8/2
= 4
Ammonia NH3:
Central atom is N. There are 3 Hydrogen atoms.
Valence electron = 5
Add
these, 5+3 = 8
Number of bonds (N/2) = 8/2 = 2
. . . Shape is Triangular pyramidal.
PCl5:
Central
atom is P. There are 5 Cl atoms.
Valence electron = 5
Number of bond (N/2) = 10/2 =
5
Bonding electron = 5, Non
Bonding electron = 0
. . . Shape is Triangular bipyramidal.
CIF3:
Central Atom is Cl.
Valence electron = 7
There three fluorine
atoms, so the number of electron pairs shared is = 3
Sum is 10 No. of bond =
10/2 = 5
Shape is T-shaped.